Abbott laboratories
Elenco abbott laboratories
-

Phytorelax laboratories cocco vegan & organic latte corpo 250ml phytorelax laboratories cocco vegan & organic latte corpo 250ml la lozione ha straordinarie proprietà emollienti, lenitive e nutrientisolo per uso esterno, non ingerireapplicare sul corpo con leggeri movimenti massaggianti fino a completo assorbimentodi facile assorbimento, rende la pelle elastica e luminosa
Italia
6989999771118164 €
-

Our solutions support over clinical laboratories worldwidewe are looking for candidates with: robust experience in verification frameworks; demonstrated experience in formulating, executing, and documenting test procedures experience in sw testing and/or sw programming (python, c, c#, java preferred) experience with automated testing technology and implementation ability to pick up new technologies easily and quickly excellent troubleshooting skills knowledge on agile practices nice to have: knowledge of canopen communication protocol; experience with test frameworks (such as robot framework) in a ci/cd environment - jenkins, bitbucket, kubernetes; knowledge of git or other software versioning applications; knowledge of devops continuous integration (jenkins, cloudbees, etc…) knowledge and practice on industrial automation systems and medical devices; what we offer: a dynamic work environment where you can make a difference and grow your career; smart working 4 days per week; company restaurant for the lunch break; agreements for bank account, credit card and personal purchases; opportunity of professional and salary growth through various initiatives: annual performance appraisal in order to evaluate the achievement of individual objectives; inner job posting to apply for career opportunities in inpeco, even between different locations; annual salary review linked to performance and professional development; continuous training on the job, through meetings or conferences, seminars and eventswould you like to work in a technological company dedicated to the development of medical solutions? inpeco is the world leader in the automation of clinical laboratories, where we create innovative robotic solutions to manage biological samplesour systems reduce the possibility of human error, limit the risks of contamination and guarantee complete traceability of the samplethe inpeco group has its headquarters in novazzano (switzerland), a production plant in val della torre (torino), a site dedicated to innovative projects in pula (sardinia), a small site in verona and two reference locations for the foreign market in brussels (belgium) and new jersey (united states)key responsibilities: cooperate to design products with high focus on quality in terms of performance, robustness, re-usability, serviceability and maintainability of the solutions, driving verification test campaigns; formulate, execute and document tests at software component level; runintensive functional/non_functional testing, performance, stress testing; collaborate daily with the developers analyzing each user stories and implementing proper tests; cooperate with other team members and project managers in order to deliver best in class products driving the best quality at the right timewhat are you waiting for? come join the inpeco team and innovate with us! for our software test team based on val della torre, torino, we are looking for a software test engineer
-

Our solutions support over clinical laboratories worldwidecapability to provide sustainable solutions in compliance with regulation/standardswhat are you waiting for? come join the inpeco team and innovate with us! for our quality department, in our company site in novazzano, switzerland, we are looking for a quality assurance engineer sr who is responsible for: support the company conformity to the fda 21cfr part 820 regulation and to the medical device european directive(s)/regulations and following quality management systems standards: iso support the company for environmental, health & safety management system: iso support the company on designing and improving the cross-functional processes support the management review process and enterprise risk management draw up procedures and instructions for integrated management systemspartecipate to the continuos improvement projects and compliance projects (egood communication skills capability to manage the conflicts english b2 level what we offer: a dynamic work environment where you can make a difference and grow your career; an international work location in our headquarter in switzerland (novazzano); home office; flexible working hours; working time account: possibility to accrue additional hours of free time in addition to holidays; additional contribution to the pension fund; professional and non-professional accident insurance; company restaurant for the lunch break; car pooling; incentives for mobility by public transport; agreements for bank account, credit card and personal purchases; opportunity of professional and salary growth through various initiatives: annual performance appraisal in order to evaluate the achievement of individual objectives; inner job posting to apply for career opportunities in inpeco, even between different locations; annual salary review linked to performance and professional development; continuous training on the job, through meetings or conferences, seminars and eventswould you like to work in a technological company dedicated to the development of medical solutions? inpeco is the world leader in the automation of clinical laboratories, where we create innovative robotic solutions to manage biological sampleswe are looking for a candidate with these skills: project management methods iso ivdr knowledge/experience 21 cfr 820 audit techniques capability to summarize and discuss critical matters with middle level managers analytical critical problem setting and solving skill capability to independently manage projects or problemsour systems reduce the possibility of human error, limit the risks of contamination and guarantee complete traceability of the samplethe inpeco group has its headquarters in novazzano (switzerland), a production plant in val della torre (torino), a site dedicated to innovative projects in pula (sardinia), a small site in verona and two reference locations for the foreign market in brussels (belgium) and new jersey (united states)manage the capa, process non-conformities and enhancements support the quality team during internal audits and the third and second party audits
-

Tasca posteriore con zip e dettagli riflettentiorli elasticizatio e a contrasto e logo sul pettogiacca da uomo realizzata in poliestere,leggeroidelae per il ciclismo con colletto alto e chiusura con zip frontale
Italia
27989999771118164 €
-
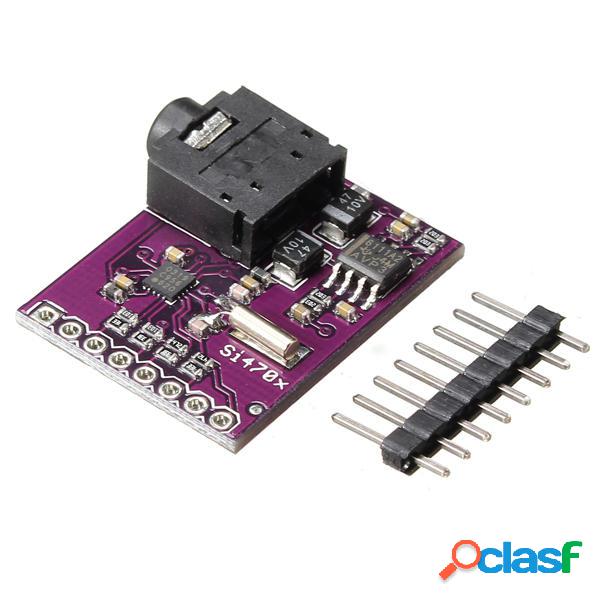
Descrizione:questa è una scheda di valutazione per il chip di sintonizzazione fm silicon laboratories si4703 che include un amplificatore e un jack per cuffie, ed è tutto ciò di cui hai bisogno per iniziare! oltre ad essere una semplice radio fm, lsi4703 è anche in grado di rilevare ed elaborare sia le informazioni radio data service (rds) che radio broadcast data service (rbds)la scheda rompe tutti i pin principali e semplifica lintegrazione di questo fantastico chip nel tuo prossimo progetto radiolsi4703 fa anche un ottimo lavoro di filtraggio e rilevamento del vettoreconsente inoltre di visualizzare allutente dati quali lid della stazione e il nome del branoinoltre, collegando le cuffie al jack audio da 3,5 mm, si utilizza efficacemente il cavo delle cuffie come antenna! pertanto, questa scheda non richiede unantenna esterna se si utilizzano cuffie o un cavo audio da 3,5 mm più lungo di 3 piediil pacchetto include:1 x scheda di sviluppo valutazioneusando questa scheda siamo in grado di captare più stazioni, così come con una radio fm standard
Italia
6 €
-

The business line electrical operates with a global network of laboratories and provides testing, inspection, and certification services to clients worldwideread and determine the applicability of national/international codes and standards for the product being tested and/or evaluated becoming a reviewer to check and support other engineerstraining other colleagues to strengthen the capability of the team participating in the technical committee, local and internationalwill be responsible for executing the medical projects according to the applicable standards in coordination with the reviewers and technical staff to help the client to reach compliancecommunicating with clients to discuss projects, technical issues found in the investigations, explain the applicable standards requests, and intertek procedurebasic qualification bs / ms electrical/electronic/biomedical engineering (or similar field) 3+ years of experience in the medical field, production or testinggood knowledge of the following standards: iec and collateral medical safety standards ability to make technical decisions and engineering judgments independently within established parameters desire to work in a fast-paced environment ability to resolve complex issues excellent written and oral communication skills good written and oral communication skills in englishsupport sales department determining project scope, evaluating the activities to perform, defining prices and timingwhere smart working and partial laboratory presence in north east italyintertek, a leading provider of atic (assurance, testing, inspection, and certification) services, is looking for a senior medical project engineer to join our italian teamwe assist manufacturers across a wide range of industries, including lighting, householding, renewable energy, professional food equipment, medical, industrial, life safety/security, it and telecom, and many more
